|
|
Hydrogen storage in magnesium based amorphous alloys
The energy needs of developing societies are increasing by the day, while our non-renewable energy sources are already dwindling and causing damage to our environment. It is urgent to provide energy with ever greater capacity in a more efficient way and through environmentally friendly techniques. An important issue is the energy supply for our means of transport, which is currently still based on the use of fossil fuels. A possible solution lies in the use of hydrogen (H). It is an excellent energy carrier and it is environmentally friendly. But there are still significant barriers to its use.
|
|
 |
|
|
 |
|
One obstacle is the proper storage of H. Currently one of the most promising ways to store it economically is to bind it in a suitable solid. Carbon nanotubes, graphene or certain metal alloys and compounds could provide a good basis for this. The most important aspect is their capacity, but many other parameters must be taken into account. The temperature and rate at which hydrogen is absorbed and released are crucial. The hydrogen adsorption-desorption process must be stably reproducible over hundreds of thousands of storage cycles. The material and production costs of the technique cannot be neglected either.
|
|
|
|
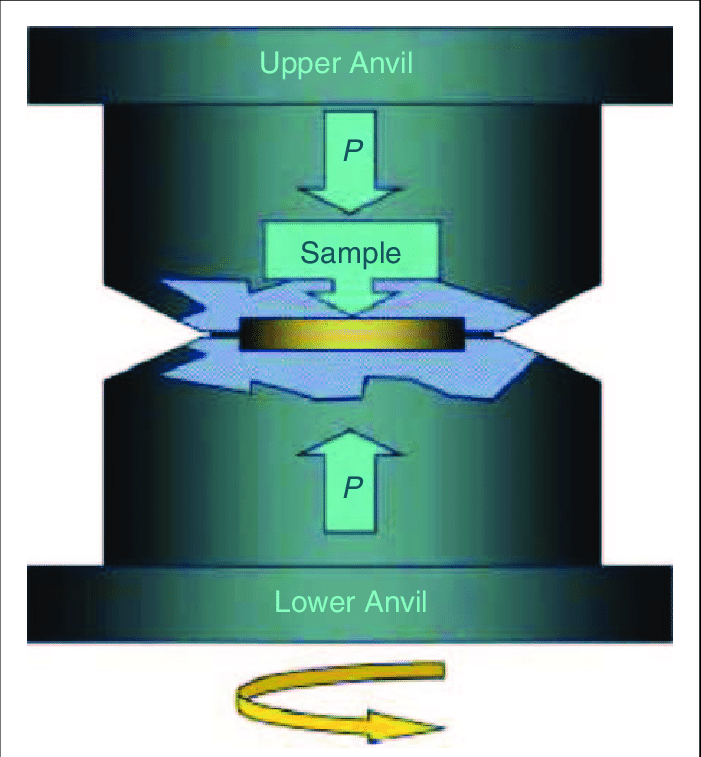
Magnesium (Mg) is one of the most promising candidates for storage. Although Mg alone is not suitable for everyday use, its properties can be improved by mechanical alloying. In the case of a metal or a metal alloy, the nature of H sorption is largely determined by the size of the crystal grains, the volume fraction of grain boundaries and the density of crystal defects in the sample. The H uptake-discharge parameters can be drastically improved if the particle size in a powder sample is reduced to the nanometre scale, while the volume fraction of grain boundaries increases enormously. Further progress can be made with further degradation, like techniques based on high ductile deformation. One such method is the high pressure torsion (HPT), where the deformation is achieved by twisting the sample between two anvils. Increasingly reducing the size of the crystalline grains can have a positive effect on H binding, so the question arises as to what happens in the case where there are no crystalline grains at all, when the system is amorphous.
|
|
 |
|
|
 |
|
We used Mg based rapidly quenched amorphous metallic glasses for our research. The samples were subjected to HPT, which resulted in a deformation dependent microstructure. Based on high-resolution X-ray diffraction, transmission and scanning electron microscopy, and calorimetry results, we found that: The H uptake in a fully amorphous alloy occurs at a significantly lower temperature compared to the fully crystallized state. While maintaining the low adsorption temperature, H storage capacity can be remarkably increased by HPT. Due to the deformation, small Mg2Ni crystalline granules are formed in the initially completely amorphous matrix, and it causes the sequestration of greater amounts of hydrogen.
|
|
Publications
|
★ High glass forming ability correlated with microstructure and hydrogen storage properties of a MgCuAgY glass
2014, Á. Révész, Á. Kis-Tóth, L.K. Varga, J.L. Lábár, T. Spassov, Int. J. Hydrogen Energy 39 (2014) 9230-9240
★ Hydrogen storage, microstructure and mechanical properties of strained Mg65Ni20Cu5Y10 metallic glass
2013, Á. Révész, Á. Kis-Tóth, P. Szommer, T. Spassov, Materials Science Forum 729 (2013) 74-79
★ Hydrogen storage of melt-spun amorphous Mg65Ni20Cu5Y10 alloy deformed by high-pressure torsion
2012, Á. Révész, Á. Kis-Tóth, L.K. Varga, E. Schafler, I. Bakonyi, T. Spassov, Int. J. Hydrogen Energy 37 (2012) 5769-5776
|
|
|---|






